BioLyo is a non-GMP Contract Development Manufacturing Organization (CDMO) that focusses solely on providing process and analytical development services for Live Bacterial Products (LBPs). LBPs are products where the active ingredient is a living bacterium. The manufacturing process for an LBP is technically not challenging and generally follows the same steps as indicated in Figure 1. However, establishing the conditions for each of these steps, notably the medium, fermentation conditions, timepoint of harvest and the lyophilization conditions varies from organism to organism.
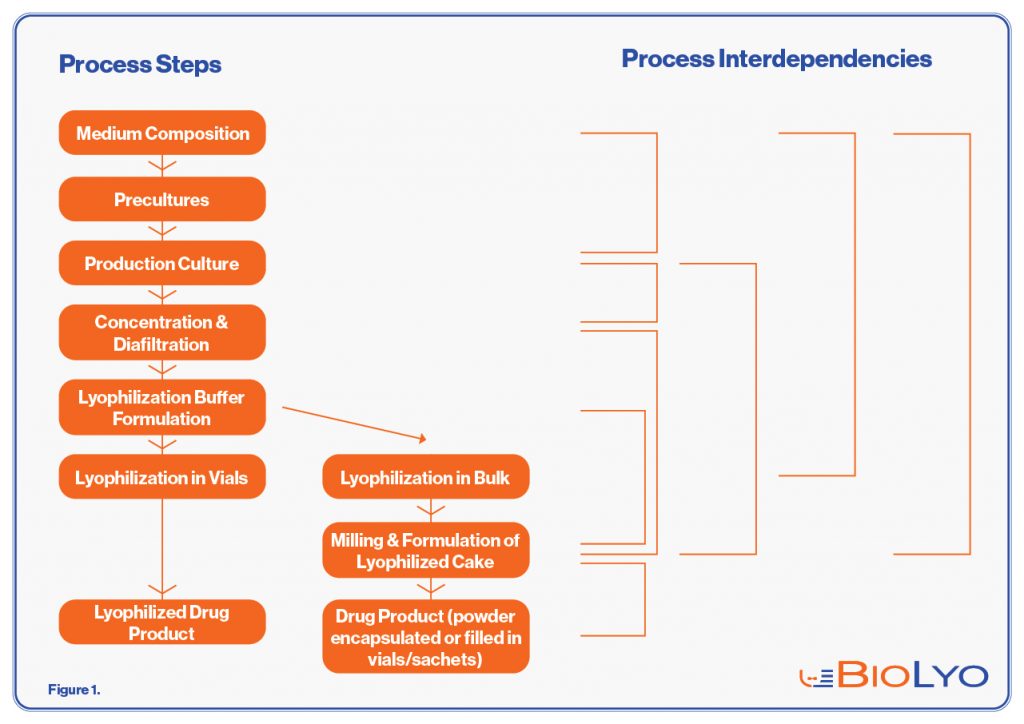
Figure 1. Flow chart of a generic LBP production process for either lyophilization in vials (left column, 1 vial = 1 dose) or bulk lyophilization (middle column), where the cake needs to be processed further to powder that can be encapsulated or filled in vials or sachets. The right column shows which process steps are or may be interdependent.
Since LBPs are a fast growing new class of products, only a limited number of CDMOs have experience with this type of product. Since CDMOs are generally relatively small organizations offering a wide range of services, a thorough understanding of microbial metabolism and how this influences survival after lyophilization is usually lacking, let alone experience with how each of the process steps shown in Figure 1 impact the resulting Drug Product. In other words, what is lacking is an integrated approach to process development, focusing on developing a process where each of the process steps are geared towards maximal survival and stability after lyophilization. BioLyo approaches LBP development (medium, fermentation, formulation, lyophilization, drug product formulation) in an integrated manner, respecting the interdependency of the process steps involved.
Although BioLyo does not yet offer GMP manufacture until 2024, all activities are performed under a QA regime since it is vital that all results, including the critical parameters identified during the process characterization, can directly be transferred to a GMP environment. Therefore all equipment and analytical methods are qualified.
BioLyo’s major strength is the in depth understanding of all aspects of live biological process development and large scale GMP manufacture so as to deliver a robust, scalable process that results in high survival after lyophilization, a Drug Product shelf life of 2 years or more and a process that can be transferred directly to a GMP CMO, if necessary under supervision of BioLyo. The rational and integrated process development approach BioLyo takes will result in a highly transparent and modular workflow that will lead to time and cost savings for BioLyo’s clients, allowing them to get to the clinic faster, at a lower cost.
BioLyo is a fast growing CDMO with a bright future since we are working on Live Bacterial Products such as live vaccines for whooping cough, pneumonia as well as on a number of products in the microbiome space that consist of consortia of strains. LBPs have significant advantages over more traditional products:
– Live (attenuated) bacterial vaccines: for respiratory tract infections for example the systemic immunity elicited by most injectable vaccines typically have no effect on colonization in the upper respiratory tract, even though all registered vaccines prevent disease. Even fully vaccinated individuals can be colonized in the nose by pathogens like B. pertussis and be transmitted to vulnarable individuals. A live pertussis vaccine being developed by Iliad Biotechnologies has shown clinical evidence to not only prevent disease but also eliminate colonization of the nose, thereby likely preventing transmission. In other words, next to preventing disease, live bacterial vaccines also have the potential to prevent transmission, the importance of which cannot be understated.
– Microbiome derived products: many of the diseases currently faced by an aging population in the US and EU turn out to be linked to or caused by an imbalance in the gut microbiome, including a number of auto immune diseases. Rather than merely suppressing the symptoms of these diseases, microbiome therapies offer the prospect of actually curing these diseases. Examples of microbiome mediated diseases are recurrent C. difficile infections, Crohn’s disease, irritable bowel syndrome and even type I and type II diabetes are being linked to the composition of the gut microbiome. ”
The BioLyo team is committed to providing fast and cost effective process development services that facilitate clinical evaluation of LBPs. Once our GMP facility becomes available BioLyo can serve as a one stop shop from cell banking to process and analytical development to GMP drug substance and drug product manufacture.